|
|
| (19 intermediate revisions by 2 users not shown) |
| Line 1: |
Line 1: |
| − | <div style="max-width:700px;margin:0 auto;">
| + | {{DISPLAYTITLE:Smartphone Chemistry Sample Cluster}} |
| − | | + | [[Chemistry sample question clusters|← Back to Chemistry Sample Question Clusters]] |
| − | == PSC Smartphone Chemistry ==
| + | {{Questionclusters |
| − | Base your answers to questions 1 through 5 on the information below and on your knowledge of chemistry.
| + | |1=Smartphone cluster q1.png |
| − | | + | |text1=[[HS-PS1-1]] |
| − | === Smartphone Chemistry ===
| + | |answer1=Smartphone chemistry answer 1.png |
| − | Smartphones have become a part of our everyday lives and there is a significant amount of chemistry involved in their production.
| + | |br1=yes |
| − | | + | |2=Smartphone cluster q2.png |
| − | Most smartphone display screens are made of aluminosilicate glass. This electrically conductive glass is composed of an oxide of silicon and aluminum with sodium ions dispersed throughout the surface. Some aluminosilicate glass is made stronger by immersing the glass in a bath of molten potassium salt at 400°C. This causes some sodium ions to be replaced by potassium ions. The potassium ions are compressed in the spaces between molecules in the glass. This makes the glass harder and more resistant to breakage, but less conductive.
| + | |text2=[[HS-PS2-6]] |
| − | | + | |answer2=Smartphone chemistry answer 2.png |
| − | Since glass is an insulator, the glass screen is coated with a layer of transparent indium tin oxide. This layer is highly conductive and allows the screen to act as a conductive touch screen.
| + | |br2=yes |
| − | | + | |3=Smartphone cluster q3.png |
| − | <span id="q1"></span>
| + | |text3=[[HS-PS1-7]] |
| − | === Question 1 === | + | |answer3=Smartphone chemistry answer 3.png |
| − | Which statement describes why positive ions in the molten salt replace ions in the aluminosilicate glass, resulting in the strengthening of the glass?
| + | |br3=yes |
| − | | + | |4=Smartphone cluster q4.png |
| − | # Sodium and potassium have similar patterns of electrons in the outermost energy level and have the same number of occupied energy levels.
| + | |text4=[[HS-ETS1-1]] |
| − | # Sodium and potassium have similar patterns of electrons in the outermost energy level and potassium is larger than sodium.
| + | |answer4=Smartphone chemistry answer 4.png |
| − | # Aluminum and silicon have similar patterns of electrons in the outermost energy level and have the same number of occupied energy levels.
| + | |br4=yes |
| − | # Aluminum and silicon have similar patterns of electrons in the outermost energy level and aluminum is larger than silicon.
| + | |5=Smartphone cluster q5.png |
| − | | + | |text5=[[HS-PS1-12]] |
| − | <span id="q2"></span>
| + | |answer5=Smartphone chemistry answer 5.png |
| − | === Question 2 === | + | }} |
| − | Explain why the particulate-level structure of the glass prevents the potassium ions in the glass from functioning as a conductor. [1]
| + | <metadesc>NYS Smartphone Chemistry questions and answer key. See the answer and relevant performance expectation for each question.</metadesc> |
| − | | |
| − | ____________________________________________________________________
| |
| − | | |
| − | ____________________________________________________________________
| |
| − | | |
| − | ____________________________________________________________________
| |
| − | | |
| − | === Smartphone Recycling and Aluminum ===
| |
| − | A popular smartphone has a mass of 172 g and requires 10.32 g of aluminum to manufacture. Equation 1 represents a model of the overall chemical process used to obtain aluminum from the purified aluminum oxide.
| |
| − | | |
| − | '''Equation 1:'''
| |
| − | 2Al₂O₃ + 3C → 4Al + 3CO₂
| |
| − | | |
| − | Some states are studying ways to require tech companies to build phones that follow clear repair and recycling standards. Recycling smartphones prevents contamination of land, water, and air, which could occur when smartphones are disposed of in a landfill. Recycling can also reduce the amount of raw materials mined and the energy used to manufacture new phones. Recycling is not always cost-effective because there are not enough valuable recyclable materials within a single smartphone. Although consumers prefer thinner smartphones, they are more challenging for recyclers to take apart. Larger phones tend to have longer battery life.
| |
| − | | |
| − | <span id="q3"></span>
| |
| − | === Question 3 === | |
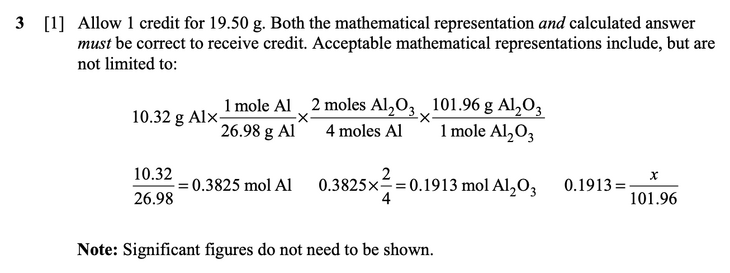
| − | Construct a mathematical representation '''and''' calculate the number of grams of aluminum oxide required to produce the aluminum necessary to manufacture one smartphone. [1]
| |
| − | | |
| − | '''Mathematical Representation:'''
| |
| − | | |
| − | ____________________________________________________________________
| |
| − | | |
| − | ____________________________________________________________________
| |
| − | | |
| − | ____________________________________________________________________
| |
| − | | |
| − | ______________ g
| |
| − | | |
| − | <span id="q4"></span>
| |
| − | === Question 4 ===
| |
| − | A criterion that should be considered when designing smartphones to be more easily recyclable is to reduce the
| |
| − | | |
| − | # battery life
| |
| − | # size of the phone
| |
| − | # amount of valuable materials in the phone
| |
| − | # disposal of hazardous components in landfills
| |
| − | | |
| − | | |
| − | ==== Figure 2: Chemical Reactions During the Discharge of a Lithium Battery ====
| |
| − | {| class="wikitable"
| |
| − | ! Equations !!
| |
| − | |-
| |
| − | | half-reaction 1 || CoO₂ + Li⁺ + e⁻ → LiCoO₂
| |
| − | |- | |
| − | | half-reaction 2 || Li → Li⁺ + e⁻ | |
| − | |-
| |
| − | | overall-reaction || Li + CoO₂ → LiCoO₂ | |
| − | |}
| |
| − | | |
| − | <span id="q5"></span> | |
| − | === Question 5 ===
| |
| − | Cite evidence from half-reaction 1 to demonstrate that there is a transfer of electrons. [1]
| |
| − | | |
| − | ____________________________________________________________________
| |
| − | | |
| − | ____________________________________________________________________
| |
| − | | |
| − | ____________________________________________________________________
| |
| − | | |
| − | </div> | |
| − | __NOTOC__
| |